Paper Authors and Title
Bienvenido Nolasco (Technological University Dublin), John Cassidy, “Kinetic study of the Copper ascorbic acid system”
Abstract
Copper (II) sulphate serves as a catalyst which increases the rate of ascorbic acid’s oxidation into dehydroascorbate.1 Literature has shown that the pKa of ascorbic acid is 4.2 2 thus at pH 5 ascorbic acid is mostly deprotonated into an electron-rich species, that reacts with the dissolved oxygen in solution resulting in ascorbyl radical (Figure 1). The delocalisation of the negative charge within the 𝜋 system stabilizes the radical, making it a good electron donor (an antioxidant).3 Reports suggest that the oxidation of ascorbic acid leads to the generation of hydrogen peroxide1. This was investigated using the Hach hydrogen peroxide analyser. Results from this analysis gave negative results for the presence of hydrogen peroxide in the solution. Figure 1 shows that the formed H2O2 can be utilised to oxidise copper into its original 2+ state. generating water in the process and hydroxyl radicals. Hydroxyl radicals formed from hydrogen peroxide through the oxidation of copper I to copper II1 are believed to react with benzoic acid.4 A kinetic study of the degradation of benzoic acid was performed by UV/vis spectroscopy at 225 nm. The results indicate that benzoic acid shows no signs of degradation under aqueous solution in the presence of copper. However, the addition of ascorbic acid caused the benzoic acid to degrade implying that benzoic acid reacted with the hydroxyl radical formed from hydrogen peroxide following the oxidation of ascorbic acid. Excess Ascorbic acid in solution hinders the degradation of Benzoic acid since the excess ascorbic acid can further react with the hydroxyl radicals.
References
- P. Zhou, J. Zhang, Y. Zhang, Y. Liu, J. Liang, B. Liu and W. Zhang, RSC Advances, 2016, 6, 38541–38547.
- M. Doseděl, E. Jirkovský, K. Macáková, L. K. Krčmová, L. Javorská, J. Pourová, L. Mercolini, F. Remião, L. Nováková and P. Mladěnka, Nutrients, DOI:https://doi.org/10.3390/nu13020615.
- G. R. Buettner and Beth Anne Jurkiewicz, Birkhäuser Basel eBooks, 1995, 145–164.
- C. Wu, A. De Visscher and I. D. Gates, RSC Advances, 2017, 7, 35776–35785.
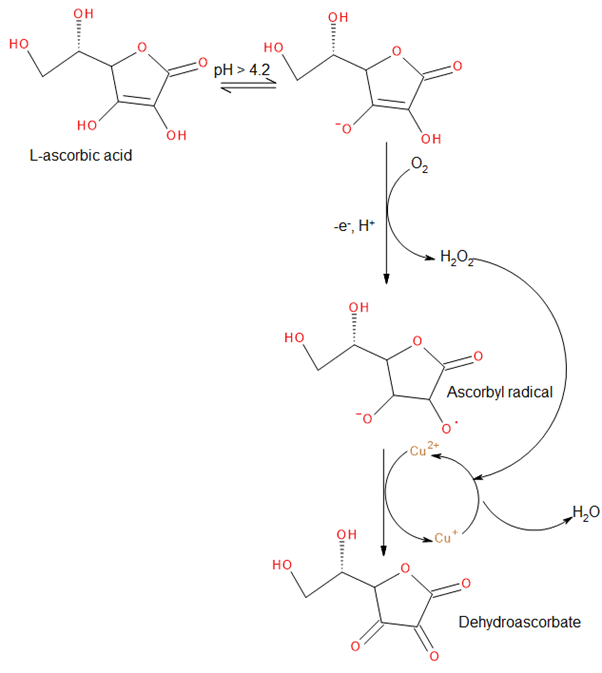
Figure 1: proposed mechanism for the degradation of ascorbic acid in the presence of a copper catalyst
